How Water Electrolysis and Ionizer Works
DEFINITION
Electrolysis is simply using electricity to cause the decomposition of water (H2O) into hydrogen gas (H2) and oxygen gas (O2).1Electrolysis was discovered in 1800 by Anthony Carlisle and William Nicholson2 and is now the primary method of mass-producing hydrogen gas for energy.3
SIMPLE EXPLANATION
| Electrolysis of pure water is very difficult,4 but adding only a small amount of ions makes the process easily achieved. In most places, there are enough minerals in the water that the ionic strength or conductivity of the water is great enough for electrolysis5 to effectively occur without needing to add additional ions to the water (this is why you don’t want to put your toaster or hair drier in water).6 | 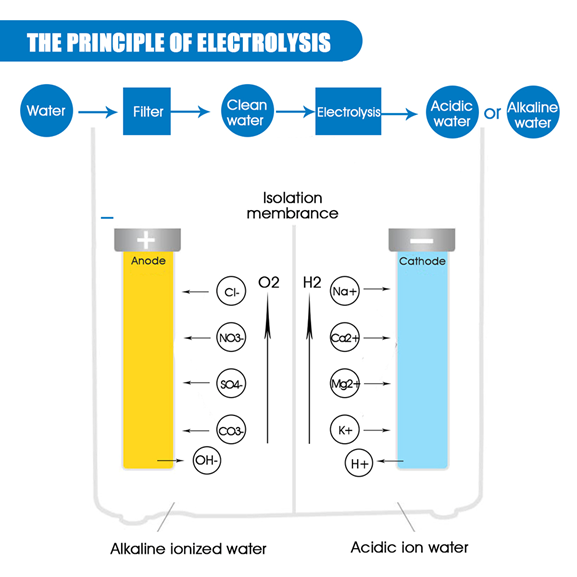 |
Water dissociates into H+ ions and OH– ions;7 the H+ ions are attracted to the negative electrode (the cathode) and are converted (reduced) to a hydrogen atoms (H) (i.e. e- + H+ => H). This is a highly unstable configuration, and therefore immediately reacts with another hydrogen atom to produce H2, molecular hydrogen gas.8
At the other electrode (the anode), oxidation occurs. The OH– ions are attracted to the positive electrode where they are oxidized to form oxygen gas (O2) and hydrogen ions (H+). However, if chloride is present, it will oxidize (instead of the OH– ions) and form chlorine gas, which will then react with the water to form hypochlorous acid.8
- The cathode reaction is: 2H+ + 2e- (cathode) => H2 (g)
- The anode reaction is: 2OH– => 4e-(anode) +O2 + 2H+
- The overall reaction is: 2H2O => 2H2 (g) + O2 (g)
INTERESTED IN THE PRODUCT - LEARN MORE | |
|---|---|
AVAILABLE IN | |
MALAYSIA&OUR ONLINE STORE | INDIA&OUR ONLINE STORE |
 |  |
A MORE COMPLETE EXPLANATION
| When current is applied to the water, the cations and anions travel to the anode and cathode respectively where they undergo either oxidation or reduction as appropriate.9 The hydronium ions migrate at high mobility via the Grotthuss mechanism (i.e. proton hopping)10 to the cathode where a hydrogen ion (H+) adsorbs to the platinum electrode11 leaving the product of water behind. The proton is reduced to its monoatomic radical species (H) and is now a singly-linked hydrogen atom that diffuses around in two dimensions until it reacts with another radical hydrogen atom to form hydrogen gas.12 The H2 gas may enter nanoscopic cavities in the liquid water13 (nano bubbles),14 or it can exist as a solvated hydrogen molecule14, para-H2 and ortho-H2,15 confined inside the small dodecahedral (H2O)20 cage16 of the sII clathrate hydrate17 (based on the coupled translation-rotation eigenstates).18 | 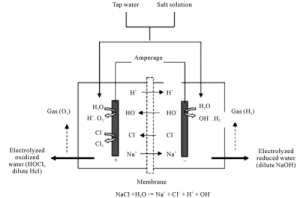 | Oxidation of anions occurs at the anode.19 Hydroxide ions (OH–) migrate via the transient hyper-coordination of the hydroxides20-21 to the anode where they are oxidized to form molecular oxygen. However, because water is a poor conductor of electricity, usually salts containing chloride (e.g. NaCl, KCl, Ca(Cl)2, etc.) have been added, or are present in tap and ground waters. This produces competing half-reactions at the anode;7 the overpotential for the oxidation of chloride to chlorine is lower than the overpotential for the oxidation of water to produce oxygen. Therefore, chloride preferentially undergoes oxidation at the anode to form chlorine (Cl2) gas, which reacts with water to form hypochlorous acid (H2O + Cl2 => 2HOCl).8 |
Reactions for electrolysis of water:
- The cathode reaction is: 4H2O (l) + 4e- (from cathode) => 2H2 (g) + 4OH– (aq)
- The anode reaction is: 6H2O (l) => 4e- (to anode) +O2 (g) + 4H3O+ (aq)
- The overall reaction is: 2H2O (l) => 2H2 (g) + O2 (g)
EFFECTS ON PH
At the anode, acid (the hydronium ion (H3O+) is produced; however, at the cathode an equal concentration of base, the hydroxide ion (OH–), is produced. Therefore, if the two compartments are able to mix together, no change in pH of the water will occur. There are small changes in the pH near the vicinity of each electrode, but once allowed to mix with the rest of the water, the pH is not altered.
If you place a membrane between the two compartments, which prevents the waters from mixing, then you will produce alkaline water at the cathode and acidic water at the anode. Because pH is a measure of the H+ ion, at the cathode the H+ ions are being converted (reduced) to hydrogen gas. The decrease in the concentration of H+ ions results in a more alkaline pH. Conversely, H+ions are produced at the anode, and so the pH is going to be more acidic. This is how most “water ionizers”/”electrolyzers”) work.
Lets get straight to point and no walk arounds
Step 1: First water is passed through the pre and internal water filter: |  |
Water coming from tap is fed through the pre filters for purification and into the ‘In” tube on the back of the water ionizer. The water runs through the in-built water filter once it is in there. Pre Filters removes most contaminants, bacteria, viruses and more and the pre-filters last for about a year
 | Step 2:Platinum Coated Titanium Plates That Perform Electrolysis |
The water ionizer undergoes a process called electrolysis wherein the water runs over positive and negative electrodes
SMPS powers the entire system |  |
Filtered water passes over the "plates” in the ionizer. Plates are part of the ionizer that does the most heavy lifting and is central to the overall ionizer.
Most ionizers use Platinum Coated Titanium plates and the size and plate design varies. Platinum coated Titanium plates are used for its non corrosion and higher electrical conductivity.
INTERESTED IN THE PRODUCT - LEARN MORE | |
|---|---|
AVAILABLE IN | |
MALAYSIA&OUR ONLINE STORE | INDIA&OUR ONLINE STORE |
 |  |

Once the water enters this section which has a very specific voltage electric current that passed through it and causes the soluble minerals in it to be attracted to either a positive ‘pole’ or a negative ‘pole’ depending on their own electrical energy signature. When this occurs, it separates the water into alkaline and acid stream.
The Alkaline Ionized water from the water ionizer is meant for drinking while the acid water is the one to be used externally, like for the skin, plants as well as for disinfecting.
 | Step 3:Alkaline and acidic water are created |
Ionization of electrodes splits water into negative ions and positive ions | 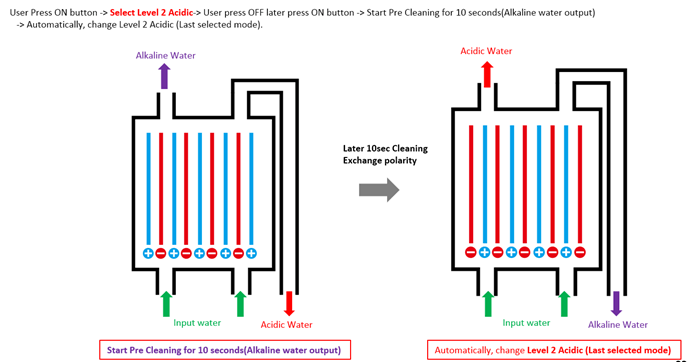 |
In this process, the water is separated into H+ (acidic water) and OH- (alkaline water). This electrolysis is also responsible for the antioxidant content ORP (Oxidation Reduction Potential) in alkaline water and for micro-clustering the water.
To make it short and simple
- The water is filtered / purified for drink ability, cosmetic purposes like taste and odor.
- The water is split into two by the ionizer
- The resulting waters are separated resulting in alkaline ionized water or acidic oxidized water.
References:
- Whitney, W. R. "Electrolysis of Water." The Journal of Physical Chemistry 7.3 (1903): 190-193.
- Andújar, J. M., and F. Segura. "Fuel cells: History and updating. A walk along two centuries." Renewable and sustainable energy reviews 13.9 (2009): 2309-2322.
- ZENG, K. & ZHANG, D. K. (2010). Recent progress in alkaline water electrolysis for hydrogen production and applications. Progress in Energy and Combustion Science 36, 307-326.
- Andreassen, K. (1998). Hydrogen production by electrolysis. In Hydrogen Power: Theoretical and Engineering Solutions (pp. 91-102). Springer Netherlands.
- Memon, Niaz A., et al. "Predictive potentiality of artificial neural networks for predicting the electrical conductivity (EC) of drinking water of Hyderabad city." Proceedings of the 12th WSEAS international conference on Computers. World Scientific and Engineering Academy and Society (WSEAS), 2008.
- Schwartz, Victor E. "See No Evil, Hear No Evil: When Clear and Adequate Warnings Do Not Prevent the Imposition of Product Liability." U. Cin. L. Rev. 68 (1999): 47.
- Day, Reuben Alexander, and Arthur Louis Underwood. Quantitative analysis. NJ: Prentice Hall, 1991.
- Harris, Daniel C. Quantitative chemical analysis. Macmillan, 2010.
- Millet, P., M. Pineri, and R. Durand. "New solid polymer electrolyte composites for water electrolysis." Journal of Applied Electrochemistry 19.2 (1989): 162-166.
- Agmon, Noam. "The grotthuss mechanism." Chemical Physics Letters 244.5 (1995): 456-462.
- Parsons, Roger. "The rate of electrolytic hydrogen evolution and the heat of adsorption of hydrogen." Transactions of the Faraday Society 54 (1958): 1053-1063.
- van der Niet, Maria JTC, et al. "Water dissociation on well-defined platinum surfaces: The electrochemical perspective." Catalysis Today 202 (2013): 105-113.
- Yang, Shangjiong, et al. "Electrolytically generated nanobubbles on highly orientated pyrolytic graphite surfaces." Langmuir 25.3 (2009): 1466-1474.
- Kikuchi, Kenji, et al. "Concentration of hydrogen nanobubbles in electrolyzed water." Journal of colloid and interface science 298.2 (2006): 914-919.
- Tikhonov, Vladimir I., and Alexander A. Volkov. "Separation of water into its ortho and para isomers." Science 296.5577 (2002): 2363-2363.
- Xu, Minzhong, Francesco Sebastianelli, and Zlatko Bac?ic. "Hydrogen molecule in the small dodecahedral cage of a clathrate hydrate: Quantum translation-rotation dynamics at higher excitation energies." The Journal of Physical Chemistry A 111.49 (2007): 12763-12771.
- Strobel, Timothy A., Carolyn A. Koh, and E. Dendy Sloan. "Water cavities of sH clathrate hydrate stabilized by molecular hydrogen." The Journal of Physical Chemistry B 112.7 (2008): 1885-1887.
- Xu, Minzhong, et al. "Hydrogen molecule in the small dodecahedral cage of a clathrate hydrate: Quantum five-dimensional calculations of the coupled translation-rotation eigenstates." The Journal of Physical Chemistry B 110.49 (2006): 24806-24811.
- Shen, Muzhong, et al. "A concise model for evaluating water electrolysis." International Journal of Hydrogen Energy 36.22 (2011): 14335-14341.
- Lee, Song Hi, and Jayendran C. Rasaiah. "Proton transfer and the mobilities of the H+ and OH? ions from studies of a dissociating model for water." The Journal of chemical physics 135 (2011): 124505.
- Tuckerman, Mark E., Dominik Marx, and Michele Parrinello. "The nature and transport mechanism of hydrated hydroxide ions in aqueous solution." Nature 417.6892 (2002): 925-929.